In recent years, the sales of apixaban have grown rapidly, and the global market has already surpassed rivaroxaban. Because Eliquis (apixaban) has an advantage over warfarin in preventing stroke and bleeding, and Xarelto ( Rivaroxaban) only showed non-inferiority. In addition, Apixaban does not require complicated dosage adjustments, and the medication is more convenient. This series of disadvantages caused rivaroxaban to lose ground in the US market.
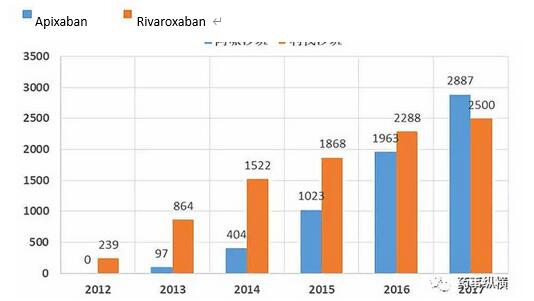
However, in mainland China, rivaroxaban has more indications globally and in mainland China. The patent expiration time of rivaroxaban is closer, and the availability of the drug will cause the population of rivaroxaban generic drugs to significantly surpass that of Apixaban.
According to Xianda data, the domestic market sales of rivaroxaban in 2018 were 2.8 billion yuan. According to the announcement of Huahai Pharmaceutical, the domestic hospital market sales of rivaroxaban in 2018 were 1.527 billion yuan.
Of course, the listing of generic drugs will lead to a sharp drop in the single-day price of rivaroxaban. Taking into account that among all NOACs, the patents for rivaroxaban compounds expire in 2020 at the earliest (the patents for apixaban and dabigatran etexilate are longer For a long time), it will undoubtedly be beneficial to the full promotion of rivaroxaban generic drugs to replace warfarin. If the daily price of rivaroxaban generic drugs is launched from 27.6 yuan to 2.76 yuan, this trend will also be strengthened.
The number of applications that only consider patients with atrial fibrillation (AF) is also amazing. The number of patients with atrial fibrillation in China is about 10 million, of which about 2 million are for valvular atrial fibrillation and about 7 million for non-valvular atrial fibrillation. Survey in 2016 It is found that the awareness rate of atrial fibrillation in Mainland China is 40%, and the anticoagulation rate is 30%. Rivaroxaban is convenient to use, does not require routine monitoring of coagulation indicators, and the anticoagulation rate will also be greatly improved. It is predicted that the generic drug of rivaroxaban will be in Mainland China. The market size used in patients with non-valvular atrial fibrillation and atrial fibrillation: 7 million patients * 30% anticoagulation rate * 50% penetration rate * 2.76 yuan/day * 365 days = 157 million yuan.
In addition, the market prospects for the prevention and treatment of VTE patients are underestimated. According to the statistics of the VTE Prevention and Treatment Center in the United States, the number of VTE patients in the United States is as high as 900,000 each year, and the number of people who die of VTE reaches 60,000 to 100,000, and there are long-term complications ( Such as DVT) patients accounted for 50%, and the number of relapses within 10 years accounted for 33%.
Due to the aging population of China, and the population base is more than 4 times that of the United States (1.395 billion people). It can be predicted that there are 3.6 million VTE patients in China each year. Assuming that the 3-month treatment rate of rivaroxaban is 80%, the market size is: 3.6 million patients * 80% treatment rate * 2.76 yuan/day * 92 days = 731 million yuan.
Therefore, only calculating the two indications of AF and VTE, the domestic market size of rivaroxaban generic drugs is close to 2 billion yuan. It is enough to cause domestic pharmaceutical companies to rush to apply for listing.
We, Changzhou Pharmaceutical Factory, also produce apxiaban and rivaroxban, and both have DMF. Rivaroxaban is under processing of applying CEP and WC.

